
While Europe has played an integral role in the development of biotech and other new pharma R&D platforms over the last 30 years, the continent has struggled to keep pace with the US, where the east and west coasts are runaway leaders in producing exciting new drug science.
Much of this failure has been not in the basis science, where Europe’s best universities are a match for those in the US, but in drawing out this science, translating it into a drug candidate with commercial potential and taking it all the way to market with strong financing and sound management.
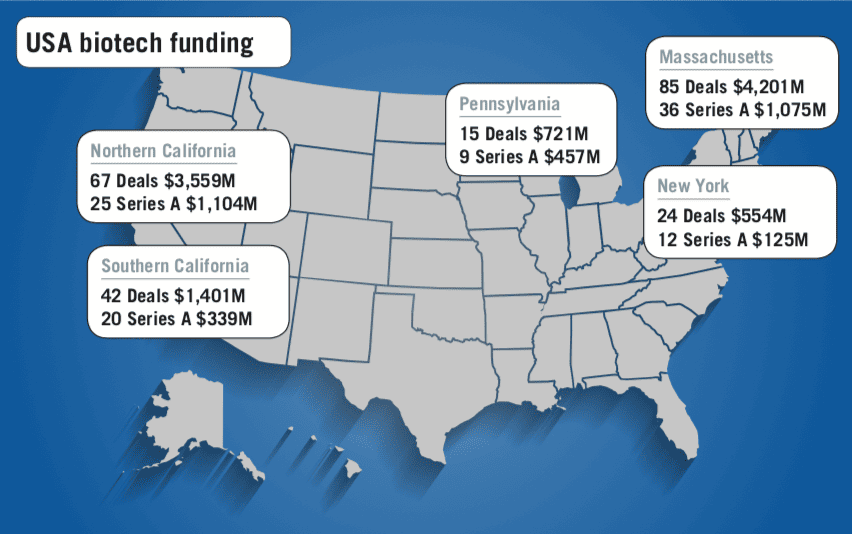
Figures from Silicon Valley Bank (SVB) for 2018 show the funding gap remains huge: the year saw biopharma investments rise to $16.23bn, with the US accounting for nearly $13.5bn of that total, and Europe just $2.7bn. Beside problems with funding and management models, one of European biotech’s problems so far has been that companies have been bought out early – illustrated last year by the $4.8bn acquisition of Belgium’s Ablynx by Sanofi.
This strategy is good news for pharma companies looking to pick up promising molecules, but of course doesn’t help build mid-to-large scale biotechs who could form a new generation and add vigour to homegrown Europe’s life sciences R&D.
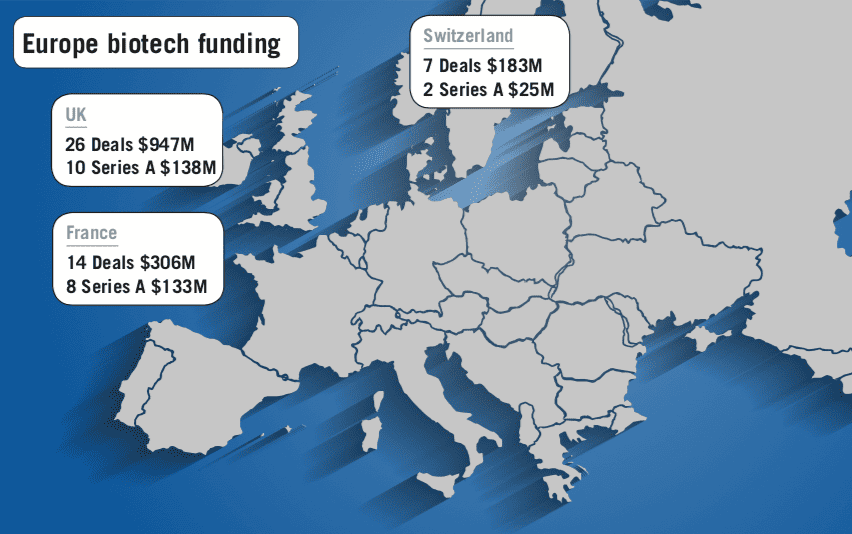
However 2019 has seen some promising signs on this front. Firstly, funding for biotech firms at all stages in Europe is growing, with specialist investors increasingly interested in tapping into the potential academic researchers across the continent, with a number of specialist venture capital firms and incubators helping to build success stories in the UK, France, Germany, Belgium and Denmark across a number of maturing biotech clusters.
Secondly, more European academic researchers are willing to become entrepreneurs in biotech spin-outs than ever before, providing local drug discovery champions who no longer have to emigrate to the US to pursue their ambitions.
Thirdly, alternative models to buying up a promising early-stage biotech company means a handful of mid-sized European biotech firms are now emerging, which could go on to be global players in the next few years.
Morphosys
One of Europe’s star performers over the last two to three years has been Germany’s Morphosys.
In April last year, it achieved a very successful IPO on the US Nasdaq, generating $208m thanks to investor belief in its cancer-focused antibody-based pipeline.
It had promising new trial data unveiled in May this year for its lead candidate the anti-CD19 antibody tafasitamab (MOR208), and an FDA filing expected by the end of this year.
The promise of the molecule is great, with analysts seeing it as a possible challenge to Novartis and Gilead’s CAR-T therapies.
The phase 2 L-MIND study in diffuse large B-cell lymphoma (DLBCL) showed the combination of tafasitamab with Celgene’s Revlimid (lenalidomide) had an overall response rate of 60%, with 43% complete responses indicating the disease had disappeared entirely.
This means its clinical outcomes could match those seen in the CAR-Ts, but without the complexity and cost of delivering these immunotherapies, and without their difficult-to-manage side effects. Unlike many other European biotechs, Morphosys isn’t seeking a marketing partner for the launch of its lead product, and aims to go it alone with the launch of MOR208. It established a US base in Boston just over a year ago, and is now building its commercial capabilities.
The CEO who has steered the company to this point, Simon Moroney, has stepped down and has handed the reins over to his successor, Jean-Paul Kress, who took up the role on 1 September.
Previously chief exec of Boston-based biotech Syntimmune, (acquired by Alexion in November last year) Kress will be in post to oversee the filing of MOR208 later this year, and its anticipated launch in 2020.
Genmab
Another big success story for European biotech is Copenhagen-headquartered Genmab. The antibody specialist company is riding high on the success of Darzalex, the multiple myeloma therapy which it has co-developed and co-marketed with J&J, and which is set to hit global revenues of $3bn this year.
Founded in 1998, the company reported $440m in sales for the 12 months ending 31 March, but will see this rise fast as royalties from Darzalex increase. It hopes to break into the big league with the launch of its own proprietary product by 2025 in addition to a string of collaborations based on its novel antibodies.
In addition to traditional antibodies, the pipeline also includes antibody drug conjugates (ADCs), bispecific antibodies created from its DuoBody technology platform, and antibodies created with its HexaBody technology. The latter is the platform used for a next-generation follow-up to Darzalex.
In June, the Danish company raised a record-breaking $506m with an initial public offering (IPO) on the US Nasdaq exchange, a move which will help fuel its ambitions for expansion over the next decade.
The listing was a record-breaker on three counts: it was the largest biotech IPO by market cap in 20 years, the largest IPO of American Depository Shares by a European healthcare company and the second largest US IPO ever by a biotech company.
CEO Jan van de Winkel couldn’t conceal his delight at the achievement on a recent analyst call.
“This tremendous accomplishment will not only help us to achieve our ambitious 2025 vision of having our own product on the markets and the pipeline of knock-your-socks-off antibodies.
“We believe that it will [also] increase Genmab’s visibility as a world class innovation powerhouse within the biotech industry and among key thought leaders and academia and in the financial community.”
Van de Winkel says the company will use its new funds to fuel its existing pipeline, but also to broaden its product pipeline and tech platforms.
That pipeline includes five candidates in clinical development and about 20 proprietary and partnered preclinical programmes, including two of its internal product candidates. Another of its partners, Horizon Therapeutics recently submitted to the FDA the Genmab-developed teprotumumab for the treatment of active thyroid eye disease. If approved, this would become Genmab’s third product on the market, behind Darzalex and Novartis’ Arzerra.
Galapagos
Another star performer this year which is breaking the mould of underperforming European biotechs is Galapagos. The Belgian-
Dutch company broke through to a new level in July this year when it signed a remarkable $5.1bn R&D partnership with Gilead, in which the star asset is its experimental JAK1 inhibitor filgotinib for rheumatoid arthritis.
For Galapagos, the partnership massively increases its financial security and firepower, while preserving its independence as a stand- alone biotech. The new funds will help accelerate its plans to transform into a commercial-stage company. This will include broader commercial rights in Europe for filgotinib, due to be filed later this year, setting up a 2020 launch.
Naturally, there remains much interest in all three of these emerging major biotech players, especially as their current market valuations fit into a ‘sweet spot’ for big pharma M&A of $5bn-$12bn.
If the leaders of these companies are determined to remain independent, and can convince shareholders of continued growth, there’s no reason why they can’t become Europe’s first ‘big biotechs’.
The growing role of specialist venture capital
One of the key ingredients in these and other recent European biotech success stories is long-term backing from investors. This include specialist venture capital firms, which are upping their investment in the region, which is seen as underdeveloped compared to the sometime overheated Massachusetts and California biotech markets.
One key venture capital fund is London-based Medicxi, which raised €400m ($450m) from investors earlier this year for its third biopharma development fund. Medicxi III was amassed in just six weeks, with corporate venture arms of Novartis and J&J Innovation among the investors along with hospital foundations and medical institutions.
It takes the total raised by Medicxi in the last three years to more than €1bn, reflecting growing appetite among investors to back early-stage European biotechs.
Medicxi’s leaders say the European life sciences sector is well recognised for its innovation, but has been held back in the past by a lack of growth capital. However they say that’s changing fast, with European companies growing in stature and offering improved returns for investors.
The new fund will be used to back projects across the full healthcare continuum, including researchers who have a great idea but lack experience of drug development.
As venture capital (VC) investors, Medicxi is focused on creating an “asset-centric” investment model, and not getting carried away with building bricks-and-mortar firms, when the risk of failure remains high. Instead it is offering funding for “fully integrated private companies with an underlying platform and/or a pipeline of assets”.
The latter is an important development, as it would allow larger companies with later-stage assets to expand without necessarily having to move to the US, Medicxi’s co-founder Francesco De Rubertis told the Financial Times.
“The size of the fund and the speed with which it was closed reflects the continued maturity of the life sciences sector across Europe,” said Medicxi.
Medicxi III will be also supported by the investment firm’s scientific advisory board, which includes senior executives from Novartis, J&J, GSK and Verily.
European biotech is benefiting from this new support to translate breakthrough science into innovative new products, but of course this comes with some big risks. Theses include pivotal clinical development decisions, growing competition in therapy categories such as oncology which squeeze market potential, and dangers in below-par boardroom strategies.
Nevertheless, European biotech has reason to be optimistic about its future, with the emergence of mid-to-large sized sector leaders likely to inspire even more confidence from investors.




