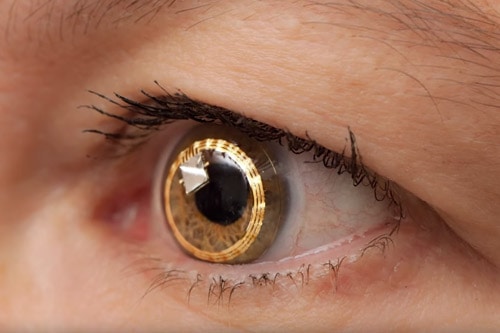
A single use contact lens that has a tiny sensor embedded to wirelessly send eye pressure data for up to 24-hours has won US approval.
The FDA granted a marketing licence to the Sensimed’s Triggerfish system, which measures tiny changes or fluctuations in an eye’s volume.
The Swiss firm’s contact lens sensor transmits data to an adhesive antenna worn around the eye, and this in turn sends the information to a portable data recorder worn by the patient.
Completing the information chain, the recorder can then transfer the data via Bluetooth to a clinician’s computer.
The FDA said the device may help healthcare professionals identify the best time of day to measure a patient’s intraocular pressure, elevated levels of which are often associated with the optic nerve damage characteristic of glaucoma.
William Maisel, acting director of the Office of Device Evaluation in the FDA’s Center for Devices and Radiological Health, said: “The Triggerfish gives the clinician 24-hour continuous monitoring of changes in IOP patterns that otherwise could not be obtained.
“This information can help determine the most critical time of day for the clinician to measure the patient’s IOP.”
Glaucoma is a leading cause of vision loss and affects an estimated three million people in the US with many suffering no symptoms until significant and irreversible vision loss.
FDA approval came after the US regulator reviewed the product through its de novo premarket review pathway, a regulatory pathway for some low- to moderate-risk medical devices that are not substantially equivalent to an already legally-marketed device.
Triggerfish is approved in Europe, where it has a CE mark, and its new US approval is for its use in adults age 22 and older under the direction and supervision of a health care professional.
Wearable sensors are attracting increasing attention in healthcare, where the front-runners include UCB, which has begun trials of a wearable sensor to monitor the symptoms of Parkinson’s disease, and Proteus’ ingestible ‘digital medicine’.
In eye care Novartis has licensed the rights to Google’s smart lens technology and is developing it for use in diabetes and presbyopia, with clinical trials due to begin later this year.




