Apple is slowing down iPhones. Volkswagen has gamed its emissions data. Google is skewing search results. Banks have manipulated foreign exchange markets. It seems like everyone is cheating these days. And the pharmaceutical industry is no exception.
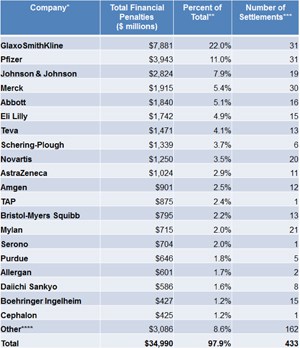 In an analysis that chronicles 25 years worth of pharmaceutical industry settlements and court judgments (see Figure 1), the group known as Public Citizen found that, from 1991 to 2015, a total of 373 settlements were reached between the federal and state governments and pharmaceutical manufacturers, for a total of $35.7bn. And this is just in the US. If we include worldwide financial penalties that have been levied against pharmaceutical companies, the aggregate number is staggering.
In an analysis that chronicles 25 years worth of pharmaceutical industry settlements and court judgments (see Figure 1), the group known as Public Citizen found that, from 1991 to 2015, a total of 373 settlements were reached between the federal and state governments and pharmaceutical manufacturers, for a total of $35.7bn. And this is just in the US. If we include worldwide financial penalties that have been levied against pharmaceutical companies, the aggregate number is staggering.
Not convinced that this happens in other jurisdictions? Well, Pfizer was fined a record £84.2m by the UK’s competition regulator after the price charged to the NHS for an anti-epilepsy drug was increased by up to 2,600%. The Competition and Markets Authority, issuing this fine, said the “extraordinary price rises have cost the NHS and the taxpayer tens of millions of pounds”. And just a few weeks ago, the EU Court of Justice ruled that an arrangement between Roche and Novartis to channel demand to an expensive drug for treating a serious vision problem breached European Union competition rules – this ruling upheld a 2014 decision by Italy’s antitrust authority that the drugmakers colluded to boost sales of Lucentis by discrediting the cheaper Avastin drug by emphasising potential side effects. Finally, in 2014, the Japanese Health Ministry filed a criminal complaint against the pharmaceutical company Novartis, calling for an investigation into the drug company’s local unit. It is suspected that falsified data was used in the clinical trial of Novartis’s best selling drug Diovan. This is most definitely a worldwide issue.
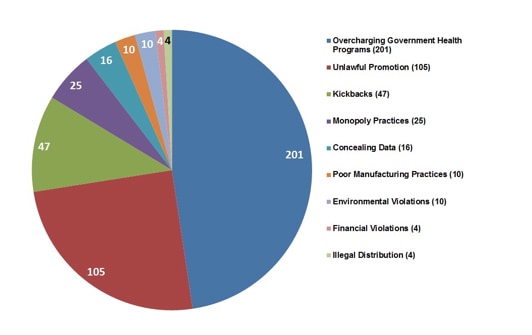
The types of ‘fraud’ run the gamut of almost everything you can think of (see Figure 2). We tend to think that pharmaceutical cheating is reserved for off-label promotion practices. But it’s not. From concealing study data to over-charging government health programmes, these transgressions are indiscriminate.
 Why should we care? After all, it doesn’t seem like there are any lasting ramifications. Pay a fine and move on. But we need to care. We need to care because we continue to erode the public trust. With an alarmingly low reputation among consumers, every time a manufacturer engages in fraudulent behaviour of any kind, we destroy the positive net social benefit that we bring to society. Making life-saving medicines is no good if you rob the public purse or falsify and distort data.
Why should we care? After all, it doesn’t seem like there are any lasting ramifications. Pay a fine and move on. But we need to care. We need to care because we continue to erode the public trust. With an alarmingly low reputation among consumers, every time a manufacturer engages in fraudulent behaviour of any kind, we destroy the positive net social benefit that we bring to society. Making life-saving medicines is no good if you rob the public purse or falsify and distort data.
From a behavioural perspective, this seems like a simple discussion. The financial penalties being imposed are not strict or severe enough to modify the behaviour. So, increase the fines, right? Make these companies pay more and more until they get it. Unfortunately, that may not work either. Without going down a rabbit hole of behavioural economic theory, let’s just agree that a quarter of a century of data doesn’t seem to support the idea that financial penalties will stop this behaviour.
So, what will? Jail time for executives hasn’t been a disincentive. Public shaming hasn’t stemmed the tide of bad behaviour. Maybe we should knock some time off the patent life of the molecule? But this is complicated. What do we do with generic culprits where there is no patent to deal with? And even if we’re dealing with an innovator manufacturer, what if the loss of sales from a truncated patent life is less than the financial penalty? Other ideas have been floated as well. But none seems to have gained any traction.
The reality is that this continues to happen precisely because imposing any sort of meaningful penalty that will change behaviour is extremely difficult to do. And the industry knows that. These transgressions are not accidents. Hiding clinical trial data doesn’t ‘just happen’. Providing kickbacks and promoting products off-label requires concerted effort on behalf of many people. A single individual doesn’t just decide to do this while keeping his/her colleagues in the dark.
Can we look to other industries for a solution? It doesn’t appear so, as Google and Apple and Volkswagen continue to trudge along after paying their respective fines too.
It’s a pervasive and systemic societal problem. And as one senior executive in the industry told me when I asked him about his perspective: “If nothing changes, this will continue to happen.”When pressed for a rationale, he said: “Because for us, it’s only money.”




