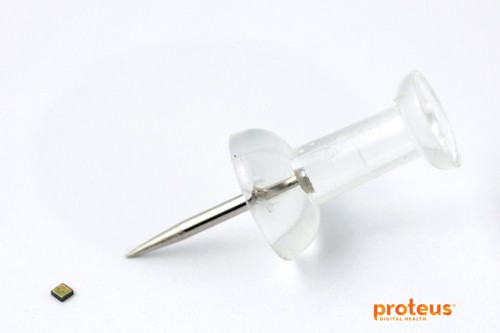
The trial will involve sensors that are about the size of a grain of sand
An industry-backed health technology company is set to begin UK trials of its ‘digital medicine’ technology that it hopes will improve the effectiveness of existing pharmaceutical products.
Proteus Digital Health’s digital medicines contain a tiny sensor that is activated in the stomach and communicates information about how an individual is taking their medication and how their body is responding to therapy.
This information is then communicated via a patch the patient wears like a plaster and the technology aims to enable more effective monitoring of patients and to help them take charge of their health.
Trials of the technology will be run by the Eastern Academic Health Science Network (EAHSN), The Northern Health Science Alliance (NHSA) and Oxford University, Oxford University Hospitals NHS Trust and Oxford Academic Health Science Network (OAHSN).
Dr Hakim Yadi, CEO of the NHSA – an alliance of universities, teaching hospitals and Academic Health Science Networks across the north of England – said: “Our lives are becoming increasingly digital, a variety of sectors have already undergone fundamental changes through the introduction of innovative digital approaches.
“Healthcare is clearly the next sector to benefit from such changes and we are delighted to be working with Proteus to pioneer the use of this technology at scale in the north of England to the benefit of patients and the NHS.”
His organisation and Proteus’ other partners will work together to better understand how digital health technology can benefit both patients and the NHS.
Unused prescription medicines are estimated to cost the NHS in England an estimated £300m and a significant area of interest for the collaboration is likely to be compliance.
Meanwhile, Proteus has also picked the UK as the location for its first manufacturing base outside the US, which will create 200 new jobs and, the firm said, serve as a hub for the global digital medicines industry.
Proteus’ funding partners include Novartis, Otsuka and Medtronic, and the California-headquartered firm has received a CE mark in Europe, and FDA market clearance in the US, for its wearable and ingestible sensor devices.




