Fuelled by changing epidemiology, reimbursement incentives and advancement of the local pharmaceutical industry, the emerging country biosimilar market has been taking an increasingly large share of the global market.
So, what lies in store for multinational pharmaceutical companies (MNCs)? What are the real opportunities and risks in entering these markets and how can the fomer be optimised and the latter minimised?
China, with a burgeoning biosimilar market, has been on top of the strategic agenda for MNCs. However, fierce local competition, lengthy registration processes and unfamiliarity with the country’s commercialisation channels have deterred many Western entrants. So, what potential strategies are needed to unlock the opportunities the Chinese biosimilar market presents in the local and global perspective while minimising imminent risks?
Shifting disease burden and increasing reimbursement pressure for innovator biologics makes China an ideal market for biosimilars.
Historical data shows 40 per cent of China’s $1.5bn recombinant biologic product sales come from biosimilars, which have enjoyed approximately 25-30 per cent CAGR over the past decade. If the market continues to grow at 25 per cent per year, the biosimilar market could grow to $2bn, around 20 per cent of the global biosimilar market, by 2015.
There are several factors driving the domestic biosimilar market:
Firstly, disease burden shifts result in relative increases in demand for biologics. China’s disease burden is shifting from the infectious diseases associated with developing countries to chronic diseases associated with diet and environmental changes, in which biologics are most commonly considered treatment choice.
Secondly, significant price discounts compared to originator products encourage reimbursements. Currently, very few of originator biologics are reimbursed in China. However, the substantial price discount between originators’ biologics and biosimilars (typically 60 per cent) has helped biosimilars to gain reimbursement from government health insurance plans, and to reach a broader customer group that cannot afford or are unwilling to pay for originator biologics.
Thirdly, a window of opportunity has been inadvertently created by today’s biosimilar leaders. As the first wave of biologic originators did not file or adequately protect its IP in China during the 1990s (nor did it actively seek to influence the regulatory environment to register products there quickly), a number of domestic players took advantage of this window of opportunity.
Several players have successfully achieved clinical approval for their biosimilar version of the products under the regulatory pathway for novel biologics.
Innovative domestic players are the winners of the biosimilars ‘boom’ while the opportunities of MNCs are squeezed due to intensifying competition and an absence of a biosimilar licensure pathway.
Besides the projected expansion of the biosimilar market in China, deep analysis of the historical to current market dynamics and country-specific policies reveals several hurdles for MNCs to consider before exploiting the lucrative biosimilar opportunities.
There is no indication that China will establish a biosimilar regulatory pathway in the next five years
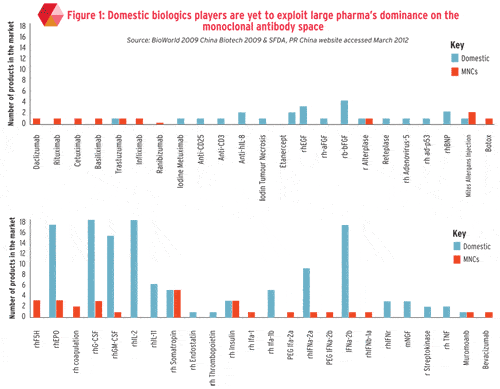
Domestic biosimilars have been marketed in China for 20 years. The high number and increasingly wide range of local offerings have left little space for new entrants. Due to the relatively low entry barriers and waves of investments, there are now over 100 biologics (excluding blood-derived products, whole bacterial products and vaccines) in China.
Most biologics manufactured by domestic players are first generation biosimilars including rhEPO, rhIFN, rhInsulin, rhIL-2, rhGCSF, rhGM-CSF, and rhGH (see graph), suggesting innovative MNCs with a complex biosimilar portfolio might have a competitive advantage. However, with thousands of highly skilled overseas-educated talent returning to the homeland every year and biomedicine being listed as one of the country’s seven ‘key strategic industries for development’ in China’s 12 Five-Year Plan, the technical and investment gap of biosimilar development between MNCs and local players is narrowing.
According to industry experts, in the next four to five years, following the patent expiration of originator biologics, a handful of second generation biosimilars such as long-acting recombinants and mAb will be marketed in China and manufactured in high quality and volume. For example, Shanghai-based CP Guojian has several mAbs which have been approved by the State Food and Drug Administration (SFDA) or are in late-stage clinical development.
Currently no specific biosimilar approval process exists in the country making the registration process highly lengthy and costly. The Chinese Revised Provisions for Drug Registrations require biosimilars to be treated as new drugs. Typically, four phases of clinical trials are required for biologic drugs even if the innovator brand is marketed in China. The entire registration process typically takes six years or longer when accounting for both clinical trial application/new drug indication (CTA/IND) and new drug approval (NDA) time.
Unlike other emerging markets such as Brazil, which are starting to follow World Health Organization (WHO) or EU guidelines on biosimilars, there is no indication that China will establish a biosimilar regulatory pathway in the next five years.
Local manufacturers also benefit from low development costs as well as government support. The average discount for the leading biosimilars in China is 60 per cent, while the average discount is 23 per cent in Europe, 20 per cent in the US and 30 per cent in Japan. Optimisation of clinical development and increasing manufacturing scale are projected to maintain the low-cost advantages of domestic players in the near to mid-term future.
While imminent hurdles listed above have caused pharma companies to debate whether the biosimilar opportunity is real and attractive in China, Deallus believes the Chinese biosimilar market could in fact present more opportunities than risks if MNCs identify an optimal route of market entry and integrate their China strategy into a regional and global mission.
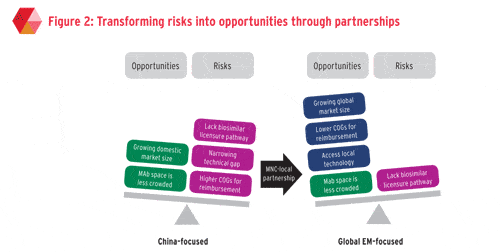
Forming strategic alliances and partnerships with local Chinese manufacturers can turn the risks of the Chinese biosimilar market into opportunities.
On a country level, collaboration with local Chinese players offers multifaceted competitive advantages and circumvents market access hurdles. Such partnerships can take different forms but trends are emerging.
Analysis of 62 deals initiated by foreign companies in China between 2007 and 2011 suggest that although the global fully-integrated companies have not built end-to-end capabilities in China yet, local CROs are expanding rapidly into the full development area. This provides an alternative opportunity for pharma to partner with local CROs to complete the local development with increased speed-to-market and potentially effective cost structure.
Conversely, any other current stakeholders specialising in different parts of the value chain, sooner or later, should be able to form the right local partnership to speed up time to market for biosimilars without de novo investment. Besides the cost benefit, partnership with a local CRO has regulatory advantages. Multinational companies often face longer drug registration timelines for their products if they are not manufactured domestically, since they are required to complete an additional step for certification of pharmaceutical product and good manufacturing practice (GMP) in China.
… biosimilars are destined to surface as a key agenda for partnerships
On a global level, partnering with Chinese biosimilar players can provide synergies across multiple functions. Other less regulated emerging markets in Asia and Latin America are also expected to have a surge in demand for biosimilars in the next five to 10 years, of which both the Western and low-cost biosimilar manufacturers anticipate a share.
MNCs that are traditionally generics players such as Sandoz and Teva can benefit from such partnerships with Chinese local players’ low cost manufacturing in order to build a good value proposition for reimbursement in other budget-restricted emerging markets.
Other MNCs that do not develop biosimilars in-house but have extensive and established presence in these emerging markets have the option of capitalising on licensing-in products or co-marketing with Chinese biosimilar players.
In other cases, strategic collaborations can include both of the above models for different product lines. However, before taking such moves, the dilemma for MNCs is whether having one product for the global market is sustainable considering the competitive market dynamics on a local level. The alternative, to manufacture market-specific or regional products, could be the most appropriate or realistic way to explore the imminent biosimilar opportunities on a global scale. However, this would inevitably compromise the reputation of MNCs for only delivering and advocating products of uniform high quality in all markets, which is a crucial differentiator versus local players in emerging markets, and it is also the basis for pricing premium.
Although many MNCs are yet to declare their intent in the Chinese biosimilar market, early movers are well-positioned to embrace the opportunities. Several joint venture deals between MNCs and Chinese players have been sealed recently: Hisun with Pfizer, Simcere with Merck and Fosun with Lonza. All of them are aimed at collaborative development, manufacturing and commercialisation of off-patent products in China and across the world.
Although the priority at this moment is chemical generics, with the waves of biologics patent expirations in China in the next few years, biosimilars are destined to surface as a key agenda for the partnerships.
Such partnerships provide a win–win situation as local players also benefit from MNCs’ expertise and capabilities. While most existing Chinese players follow a ‘China for China’ strategy, a few leading players with more advanced quality standards and manufacturing capabilities have set their sights on other less regulated emerging markets as well as developed markets.
The extensive footprints in emerging countries of MNCs in such partnerships can be leveraged to broaden Chinese players market base significantly. For leading innovative Chinese companies with sights set in the EU and US, there are substantial learning opportunities from partnering with MNCs in terms of product quality assurance, data documentation and licensure applications.
MNCs that are pondering whether to enter the Chinese biosimilar market should have a clear vision of their local and global objectives in the biosimilar business. Conducting due diligence on prominent Chinese biosimilar players while exploiting different partnership models that align capabilities and business objectives are crucial in determining the delicate balance of risk and opportunity that the Chinese biosimilar market presents.