There is no doubt that the current challenges facing pharma are driving increased pressure on financial return. The requirement for greater value from medicines in targeted patient populations demands consideration of your brand’s value proposition earlier and earlier in the product life cycle and better clinical and commercial alignment.
However, with earlier decision-making, the stakes are higher and this brings with it greater risk. How can risk be mitigated and what role can ‘real-world’ insights and evidence play in this?
Early value proposition is key
‘Traditional’ market research has long been recognised as key to informing decisions across the life cycle. Insights from qualitative research tend to inform decision-making before proof of concept, with more quantitative rigour being employed as the product moves through to Phase III and beyond. Furthermore, the development of the value proposition and market access strategy is increasingly part of this insights process, and payer needs and expectations are critical to a differentiated brand strategy.
Identifying payer unmet needs within target disease areas and patient populations (linked to product development) will be critical in building an understanding of the evidence needs to inform the combined development of relevant randomised controlled trials (RCTs) and real-world studies.
However, with the increasing need for proof-of-concept decisions earlier in the process and better understanding of potential clinical and commercial value, there is now a major role for real-world studies to both validate insights hypotheses – providing the necessary rigour – and to begin to shape the evidence needs for value demonstration further down the line.
Validating insights
Understanding patient populations will be critical in developing medicines that provide targeted value propositions for payers, ultimately leading to improved patient outcomes. Real-world data can assist in segmenting patient populations to validate early insights evaluation as to which patient types will benefit most from the proposed value proposition.
Target product profile (TPP) evaluation, and the propensity to prescribe and reimburse, is also an area where real-world insights validation provides a robust process to appraise likely uptake. By using either multi-client or bespoke prospective patient observational studies in selected disease areas, it is possible to evaluate a TPP in a ‘real-world’ setting, with the physician able to provide a view on whether or not your product is likely to be prescribed or reimbursed within a specific population. This ‘real-world’ knowledge can then be aggregated to provide robust and validated information on which patient segments to target and how to tailor the value and brand propositions – invaluable for mitigating risk in decision-making at this early stage.
Evidence for value demonstration
Real-world studies are becoming increasingly important to payers, who need to evaluate how drugs perform in routine clinical practice and not the artificial environment of an RCT. It aids optimal decision-making on how and where to use limited resources in the reimbursement of drugs.
The validation of key insights with real-world data provides a solid base to define the scope for optimal study protocol development in order to develop relevant pharmacoeconomic arguments for payers. This validation, for instance, can provide input into patient population selection, end-point measures to be included and addition of relevant patient-reported outcomes.
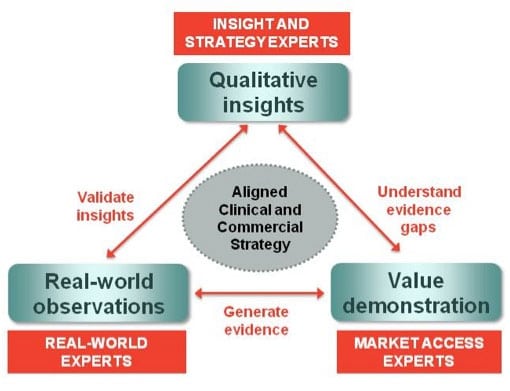
Real-world observational patient studies can be set up to generate the various types of evidence needed to support the reimbursement process. This might include cost-effectiveness, burden of illness and utility studies – all of which can provide credible value arguments as part of the evidence base.
In summary…
Quicker, more efficient, risk-minimised decision-making is the ultimate goal for companies seeking clinical and commercial alignment and commercial success. Clearly identifying the true value of your brand at an early stage and building this into brand strategy is critical to achieving this. Real-world studies offer the opportunity to lend rigour to early insights and also to provide the necessary evidence to ensure your brand resonates with your customers and returns are maximised.

 The Authors
The Authors
Suzie Denton at Complete Clarity and Andy Turner at Complete True Life
suzie.denton@completeclarity.com or andy.turner@completetruelife.com




