How is the vaccine reimbursement environment changing?
March 14, 2024 | HTA process, immunization, vaccine, vaccine reimbursement
By Claire Woon

Immunization has been shown to be one of the most successful strategies for improving the overall health of populations. In recent years, new vaccines have been launched offering protection against diseases including malaria, Ebola and dengue, demonstrating a shift in the vaccine paradigm to include a focus on diseases affecting those living in low- and middle-income countries.
The COVID-19 pandemic reminded the world about the power vaccines have to fight disease and save lives. It highlighted the speed at which safe, efficacious vaccines could be developed when supported by effective collaboration between pharmaceutical companies, governments and bodies such as the World Health Organization (WHO) and how timelines for approval and reimbursement can be accelerated. However, it also delayed well-established and effective vaccination campaigns, leaving populations less protected against diseases that vaccinations have long prevented or reduced.
In this blog post, we will discuss the recent shift in the vaccine paradigm, including drivers and unmet needs, and what further changes might look like. We will explore how pharmaceutical companies have a critical role to play in driving the use of broader elements to define value and ensure vaccine access for more people.
A life-course approach to immunization
In 2011, the WHO published a Global Vaccine Action Plan that recommended a life-course approach to immunization.1 The life-course approach recognizes the role of immunization as a strategy to prevent diseases and maximize health over an entire life, regardless of age.2
It requires immunization schedules and access to vaccination to be aligned to stage in life, lifestyle and specific vulnerabilities/risks of infectious diseases. This approach moves away from the widely accepted programmes limited to childhood vaccinations, instead taking a more holistic approach that, given the world’s ageing population, may have a wide range of benefits, including:
-
- Individual and population health improvement
- Promotion of healthy ageing
- Long-term sustainability of health systems through reduced healthcare costs
- Wider economic benefits by promoting workforce productivity
- Supporting equity and universal access to primary care by providing a platform around which these services can be provided
- Reducing the spread of antimicrobial resistance (AMR)
The life-course approach to immunization framework includes five key policy components that are needed for an effective life-course immunization policy:3
-
- Robust data collection is at the core and is a key area for pharmaceutical companies’ contributions. From vaccination trials to long-term data collection, robust evidence (including epidemiology, transmission, efficacy and long-term impacts) can support all other aspects
- Engaged healthcare professionals (HCPs) to drive uptake and support public understanding
- Public demand for immunization due to good education and awareness
- Comprehensive immunization programme to foster confidence
- Multidisciplinary and cross-sectional coordination to ensure uninterrupted supply and smooth delivery
There is an obvious need for these key components to be supported by a conducive legislative environment and long-term cross-governmental commitment. Engagement by industry in constructive dialogue with both HCPs and the public will aid these goals. We believe the development of accessible and impactful materials can support these awareness-raising activities. We advise our pharmaceutical partners on the most appropriate materials to ensure the greatest impact.
Has the approach been widely implemented?
Despite the WHO making this recommendation over 10 years ago, a life-course approach is still not implemented in many markets for a wide range of reasons. Although the benefits are well established, the upfront cost and need for more wide-ranging cost–benefit analyses may inhibit governments’ abilities (or desires) to alter their current approach. Industry may be able to support this transition by driving the conversation and incorporating the broad societal and economic implications beyond those normally considered in health technology assessments (HTAs) at different stages in life. Communications agencies often have experience in developing HTA submissions and strategic plans. This means we can advise the most effective and impactful way to implement this in a pharmaceutical company’s strategy.
Following on from the previous action plan, in April 2020 the WHO published the ‘Immunization Agenda 2030: A Global Strategy to Leave No One Behind’.4 The strategy takes the life-course approach further and focuses on morbidity and mortality benefits across the lifetime to ensure equity of access. It also outlines a new global vision to address challenges, with an aim to save over 50 million lives.
In line with these aims, a number of new vaccinations (either recently launched or approaching launch) target diseases that are more prevalent in low- and middle-income countries. Traditionally, these markets have been underserved by pharmaceutical companies launching vaccines, with vaccination rates below those of high-income markets. However, diseases such as dengue, which is on the rise globally, often have the highest incidence in these markets. By developing these vaccines and supporting the implementation of routine immunization programmes, these outbreaks may be prevented or their impact reduced. The launch of new vaccines in these markets aligns with the life-course immunization drive owing to the need to vaccinate demographic groups beyond children and adolescents. As such, the conversations around these vaccines need to be broader, encompassing the macroeconomic and wide-ranging societal benefits, as well as more traditional metrics.
However, the pharmaceutical industry needs to recognize that they must work with country stakeholders early in the development programme to fully understand the current barriers to life-course immunization (beyond cost). By utilizing this early engagement, vaccines can be developed and trialled to fit with existing vaccination schedules, religious requirements and other country- or region-specific needs. In our experience, these types of data are key in attaining broad reimbursement and inclusion in National Immunization Programs. We can advise and support the inclusion in key materials. In addition, we recognize it is important in these markets to train local teams how to effectively communicate these less common data types. We recommend and deliver locally tailored training right from the strategy to the tactical deliverables.
How does this change current economic evaluation methods?
To support this wider approach to vaccination, the economic evaluation of vaccines must also be reassessed. Evaluations have traditionally focused on a relatively narrow set of vaccine benefits, such as cost offsets to the healthcare system. However, further vaccination benefits have been identified that should be incorporated into economic evaluations to fully reflect a vaccine’s value. Estimates of the magnitude of these broader benefits suggest that vaccination may have been substantially undervalued, which has important implications for public and private vaccine policies and provides broader societal benefits.
Bell et al. assessed HTAs across nine developed markets, showing significant disparity between the levels and/or types of evidence considered (Figure 1).2 Narrow health effects and health system economic effects are recognized in most current vaccine HTAs (impact on patient’s quality of life [QoL], length of life and cost-offsets to the healthcare system).2 Limited markets also look at broad health effects (including caregiver QoL, AMR prevention and societal equity), and very few consider societal economic effects, including patient and caregiver productivity and macroeconomic effects. In fact, macroeconomic effects are only considered by the USA, and this consideration is currently informal.
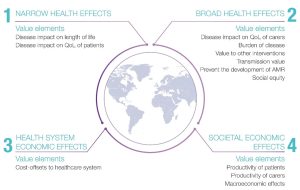
Figure 1. Value elements considered by country2
Bell et al. have termed their expert-informed, consensus-based recommendations the ‘BRAVE way forward’, which is a three-pronged approach, including:2
- The collection of high-quality evidence
- Improvement of technical and analytical ability within HTA and infectious disease modelling
- Engagement with all stakeholders involved to generate willingness to change
These three areas should be addressed in parallel rather than in isolation and can involve, or even be driven by, vaccine manufacturers. Where both willingness and evidence hurdles exist, they may be most effectively overcome simultaneously, as efforts to improve the available evidence base around the impact of vaccines may also generate willingness to open discussions on the decision maker’s side.2
By taking a holistic life-course approach to vaccination and considering the benefits to both the individual and wider society, vaccines have the potential to positively change the health and equity of society. The implications of this are wide-ranging and may include economic benefits for healthcare systems, improvements in societal health and wellbeing, and positive macroeconomic impacts. While pharmaceutical companies cannot influence the HTA process or change policy, by generating the appropriate evidence and providing data outlining these broader benefits, they can help to drive the conversation. Early engagement with stakeholders to ensure understanding of the broader burden associated with these communicable diseases will help to prepare markets for the benefits future pharmaceuticals can have to change population health for the better.
To see how we can help you build these broader value elements into your pharmaceutical strategy, submissions and materials, get in touch with claire.woon@amiculum.biz. Let’s pave a ‘BRAVE new way’ for vaccine reimbursement, together! For more insights, visit the AMICULUM blog site.
This content was provided by AMICULUM





